Fluorinated Azaacenes: Efficient Syntheses, Structures, and Electrochemical Properties
Authors: Marc Zeplichal, Joshua Gies, Johannes Bernd, Dilan Kancious Winslaws, Tieyan Chang, Yu-Sheng Chen, Steven H. Strauss, Olga V. Boltalina, Andreas Terfort
Highlights
- The perfluoroalkylation of phenazine as representative electron-poor arene was studied by thermal and photochemical transformations.
- Various side reactions in the thermal as well as the photochemical reactions could be identified and avoided by choosing optimized conditions.
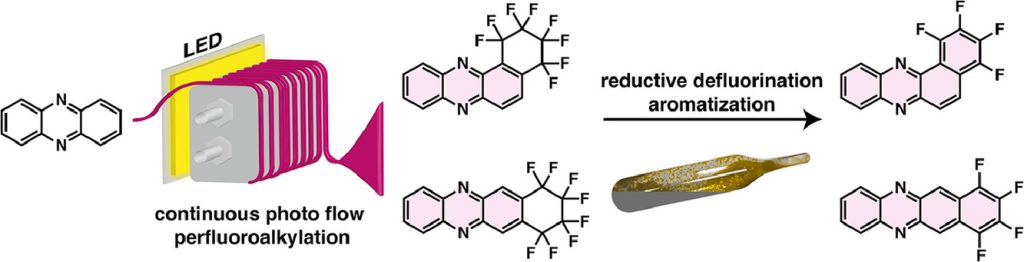
- Perfluoro-cyclohexyl systems were obtained by using 1,4-C4F8I2, which could be partially defluorinated to the respective tetrafluorobenzo systems.
- All molecules could be isolated and characterized spectroscopically and electrochemically. The perfluoroalkyl derivates show significantly increased electron affinities, while the partially defluorinated system show lowered band gaps.
- Four of the molecules could be characterized by X-ray diffraction.
Abstract
Perfluoroalkylation is an effective strategy for lowering the valence orbital energies of arenes and is therefore used for the design of new n-type semiconductors. Here we present a strategy for the efficient reaction of the diazaarene phenazine (PHNZ) with 1,4-C4F8I2, which finally leads to the cyclization products 1,2- and 2,3-PHNZ(c-C4F8) (c- = cyclo-substituent). It turns out that the synthesis of these compounds can be attained with equal efficiacy by thermal reactions (in the presence of copper) or photochemical reactions (in the presence of photocatalysts, PC). While under the photochemical conditions, the reaction either stops after formation of a 1:1 mixture of cyclo-products with the mono-substituted compounds 1- and 2-PHNZ(ω-C4F8I) (ω- = chain with terminal substitution, PC: Ru(bpy)3Cl2) or even becomes reverted (PC: Rose Bengal), the thermal reaction is limited by the formation of the reduction products 1- and 2-PHNZ(ω-C4F8H). Attempts to cyclize 1- and 2-PHNZ(ω-C4F8I) thermally led to a new cyclic compound, 5-H-1,10-PHNZ(c-C3F6C(=O)), a cyclic amide derivative of 5,10-dihydrophenazine. Nevertheless, under photochemical conditions, the purified 1- and 2-PHNZ(ω-C4F8I) could be cyclized cleanly to 1,2- and 2,3-PHNZ(c-C4F8). The cyclo-compounds could be reductively defluorinated under formation of a new aromatic ring, thus extending the π-systems to 1,2- and 2,3-PHNZ(c-C4F4), which are derivatives of benzo[a]phenazine and 5,12-diazatetracene, respectively. All these compounds could be isolated and were characterized by all or a subset of the following physicochemical techniques: 1H and 19F NMR; GCMS; cyclic voltammetry; UV/VIS spectroscopy, high-resolution mass spectrometry, and powder or single-crystal X-ray diffraction. The pronounced electron-withdrawing effect of the perfluoroalkyl groups could be verified, while the introduction of the (c-C4F4) annelation led to a significant lowering of the band gaps. The diazatetracene derivative exposed a tendency for the [4+4] cyclodimerization to [2,3-PHNZ(c-C4F4)]2, which was also isolated and structurally characterized.
Further Information
Link to paper: https://doi.org/10.1016/j.jfluchem.2022.109960